





 Dec 20, 2003: Update: I found there is one more project named
Cheaposcope;
Dec 20, 2003: Update: I found there is one more project named
Cheaposcope;
it's located at Plant Science Department of University of Cambridge, UK. They use better microscope, CCTV cameras instead of a digital still camera, 5-watt LEDs instead of a clip-on light, optical filters for fluorescence microscopy (I don't have the stains for that or I'd try it), and higher budget, all of which I admit to envy them for. I may try fluorescence microscopy too, as my new camera offers exposition times of up to 15 seconds, allowing for very low-light shots.)
Oct 15, 2007: There is another project along the lines of the original Cambridge fluorescencemicroscope, here.
Nov 8, 2007: Yet another project, homemade
@w|Two-photon_excitation_microscopy|two-photon microscope
here.
The aim of the project is to bring a cost-effective microphotography device for low-budget students, researchers, and reverse engineers.
The system was based on a 2Mpix digital camera (Sony DSC-P50), strapped onto a standard school microscope. The results turned out to be strikingly good in terms of cost effectivity. The camera lens is positioned directly on the microscope eyepiece. The optical and digital zoom are both enabled and set to maximum value (6x), which achieves the widest field of vision of the camera, further adding to the maximum magnification.
The microscope eyepiece has 15x magnification, the objectives are 3.3x, 6.7x, and 20x, offering total magnifications of the assembly being 300x, 600x, and 1800x.
The camera has a TV output, showing exactly what's on the camera's LCD in real-time. This allows using a TV or a computer monitor (using a video-in card) to have a high-resolution screen to watch the operations with the sample and to have more precise control over focus (which was difficult with the small LCD screen).
Further improvement could be using stepper motors for control of focus (Z-shift) and X-Y shift of the table, allowing automated photographing of larger samples and then assembling the large high-resolution image from the resulting tiles.
Another improvement, depending on the automatic focus, is the compensation of the low depth of focus, plaguing optical microscopy. The microscope can focus only to very small range of distances, making observation of three-dimensional samples rather difficult. A helpful approach could be making a lot of photographs with different setting of focus, layer by layer, throwing away the blurred parts of each picture, then reassembling the rest to a sharp one.
Yet another improvement would be addition of microprobes and eventually a laser, allowing for hacking of eg. electronic chips, adding micromanipulators for building extremely small devices, etc; my micromech-fu isn't good enough for this. Yet.
Selected results follow (click on thumbnails for full-size image):
Am386DX CPU. 300x and 600x magnification, reflective light. Surface contaminated by dust.
 |  |  |  |
 |  |
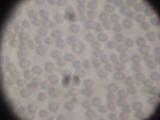
Human blood. 1800x magnification, transmissive light, specks of dirt on the eyepiece. Due to suboptimal optics, the image in this resolution isn't as sharp as I would like. The red blood cells have an average diameter of 7.5 micrometers.
 |  |
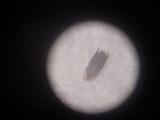
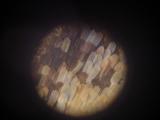
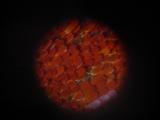
Details of a butterfly wing. The wings are covered by tiny scales of various color. The patterns on the wings are caused by various distribution of color scales, like a tiny mosaic.
 |  |  |  |
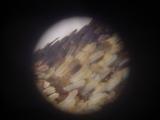 |  |  |
Detail of a LED chip on a deceased HDLG-2416 display. Reflexive light. Surface contaminated with some hard-to-clean fine dust.
 |  |  |  |
Human hair. 600x magnification, reflective light.
 |
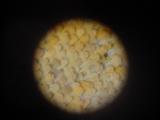
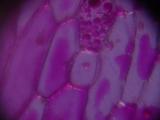
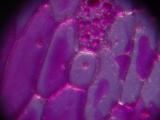
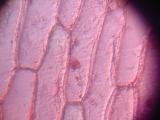
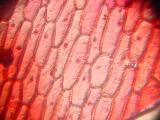
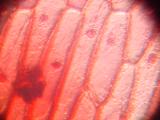
Cells of onion skin, a single-cell layer on the surface of onion layers. Classical school experiment; I remember, over 15 years ago, to tediously draw the cells with a pencil and thinking about a better way to document them. Image 3 shows multilayered cells of inner parts of the onion; note the difference between the elongated outer cells and spherical inner ones. Images 4 and higher are the result of an attempt to stain the sample with Neutral Red.
 |  |  |  |
 |  |  |  |
 |